How to Create FTC/FDA Compliant Healthcare Content: The Complete 2025 Guide

Healthcare content has never been more visible, more consumed, or more heavily regulated than it is today. Search engines, AI tools, and consumers increasingly rely on online health information to guide decisions about supplements, diagnostics, treatments, and lifestyle changes. This creates a major responsibility for brands: your content must be factual, safe, transparent, and compliant with federal regulations.
Both the U.S. Food & Drug Administration (FDA) and the Federal Trade Commission (FTC) monitor how health information is communicated. Even small wording choices can turn an educational article into an illegal medical claim.
This guide gives you a practical, step-by-step system for creating FTC/FDA compliant healthcare content, supported with direct links to authoritative U.S. government sources.
*Legal Disclaimer (Not Legal Advice): This article is for informational and educational purposes only. It is not legal advice. For specific regulatory guidance, consult a qualified FDA/FTC attorney or compliance specialist.
Why Compliance Matters in Healthcare Content

Healthcare content is treated differently from general education or lifestyle content because it influences decisions affecting a person’s body, safety, and medical care. The FDA evaluates health information to determine if it includes drug claims, while the FTC evaluates whether claims are truthful, not misleading, and properly substantiated.
Failing to follow these rules can result in:
- FDA or FTC warning letters
- Product reclassification as an unapproved drug
- Mandatory content removal
- Civil penalties and fines
- Forced refunds
- Ongoing monitoring of marketing claims
- Reputational damage
Regulators don’t just examine advertisements—they also review blogs, emails, landing pages, social content, videos, patient handouts, webinars, and educational materials.
Below are nine strategies to stay compliant, plus a rewriting table and SEO tips to guide you in staying compliant with your content.
1. Understand How the FDA Regulates Health Claims
The FDA enforces laws that determine what you can and cannot say about ingredients, supplements, foods, lifestyle interventions, tools, and diagnostics.
Under U.S. law, the FDA defines a drug according to the claims made about it, as stated in the federal definition of a drug under 21 U.S. Code § 321(g)(1).
If your content suggests that a product can diagnose, treat, cure, mitigate, or prevent disease, it becomes a drug claim, even if the product is not legally classified as a drug.
FDA-Regulated Drug Claim Examples (Non-Compliant)
❌ “This supplement treats depression.”
❌ “This herb lowers blood pressure.”
❌ “Probiotics prevent irritable bowel flare-ups.”
❌ “Red yeast rice is a natural alternative to statins.”
❌ “This test diagnoses thyroid disorders.”
These statements trigger FDA enforcement because they imply a medical effect.
2. Use FDA-Compliant Structure/Function Language
The FDA provides clear guidance on allowable structure/function claims in its official page on structure/function claims.
Structure/function claims describe how a nutrient or ingredient supports normal physiological function, without implying disease treatment.
Convert Disease Claims Into Structure/Function Claims
Structure/function claims help you stay compliant while still educating readers.
3. Understand FTC Rules: Truth, Transparency & Evidence
The FTC regulates consumer-facing health content, ensuring all statements are:
- Truthful
- Not misleading
- Backed by competent and reliable scientific evidence
The FTC’s standards are outlined in its official resource on dietary supplement advertising and broadened further in the Health Products Compliance Guidance.
FTC High-Risk Violations Include:
- Overstating study findings
- Using animal or in vitro studies to imply human benefits
- Exaggerating scientific certainty
- Implying typical results based on small or weak studies
- Suggesting benefits unsupported by evidence
- Using testimonials that imply medical outcomes
FTC-Compliant Research Language
Use neutral, cautious phrasing:
✔ “Research suggests…”
✔ “Evidence indicates…”
✔ “In one clinical trial…”
✔ “Early findings show…”
✔ “More research is needed…”
Avoid language that implies proven effectiveness unless you have strong clinical data that meets FTC requirements.
4. Avoid Giving Medical Advice or Diagnostic Guidance
Sharing information about health is allowed. Giving personalized recommendations is not.
High-Risk Examples of Implied Medical Advice
❌ “If you have anxiety, take magnesium.”
❌ “If your lab test shows high cortisol, use these herbs.”
❌ “If you experience these symptoms, you need this supplement.”
❌ “This protocol works for patients with thyroid disorders.”
Even without naming a disease, these statements imply:
- Diagnosis
- Treatment
- Prescription
- A doctor–patient relationship
How to Avoid Medical Advice Liability
✔ Use conditional, general language
- “This type of test may help clinicians gather information about metabolic patterns.”
- “Certain foods can play a role in promoting digestive comfort.”
- “Practitioners sometimes consider these lifestyle factors when evaluating sleep quality.”
- “Emerging research suggests this micronutrient may support healthy thyroid function.”
✔ Encourage readers to consult their clinician
- “Always review supplement options with a healthcare professional before deciding what’s best for you.”
- “Your practitioner can help interpret lab findings in the context of your overall health.”
✔ Focus on education, not instructions
Explain concepts, not what the reader should do.
- “Cortisol naturally fluctuates throughout the day and is influenced by sleep, stress, and activity patterns.”
- “Omega-3 fatty acids are known for supporting cardiovascular and cellular health.”
✔ Avoid stating what someone “should” take
- Instead of: “You should take zinc when you feel sick.” – “Zinc is studied for its role in supporting the immune system.”
5. Never Link Lab Results to Supplements
The FDA and FTC treat lab-to-supplement connections as diagnostic and therapeutic claims, which are tightly regulated.
High-Risk Lab-Linked Statements
❌ “If your stool test shows dysbiosis, take probiotics.”
❌ “If your vitamin D is low, you should supplement immediately.”
❌ “If your thyroid hormones are abnormal, use these nutrients.”
These statements imply you are diagnosing and prescribing.
Safe Alternatives
✔ “Some clinicians use stool testing to better understand gut function.”
✔ “Vitamin D plays a role in bone and immune health.”
✔ “Nutrients like iodine and selenium support thyroid physiology.”
Do not tell readers what to do with their results.
[blog-cta_component]
6. Write at a Clear, Consumer-Friendly Reading Level

The FTC emphasizes consumer readability in its guidelines on effective digital disclosures.
Rules for Compliance-Friendly Readability:
- Short sentences
- Defined terminology
- Everyday vocabulary
- Simple analogies
- Clear structure
- No unnecessary jargon
This improves comprehension and reduces misunderstandings.
7. Separate Educational Content From Promotional Content
Educational content is allowed. Promotional content is regulated. When the two blend, regulators may treat all the content as advertising.
Best Practices for Clear Separation
✔ Keep educational content ingredient-centric
✔ Keep product pages product-centric
❌ Do not hyperlink educational claims directly to product purchase pages
❌ Avoid promotional CTAs in educational articles
❌ Do not mix testimonials with clinical explanations
This prevents reclassification as labeling or advertising.
8. Include Mandatory Disclaimers
Two disclaimers are essential for compliance.
I. Medical Advice Disclaimer
Place this at the top or bottom of every health article:
- “This information is not a substitute for medical advice. Always consult a qualified healthcare professional before making changes to your health routine.”
II. FDA Supplement Disclaimer
Required under federal law and outlined in 21 CFR 101.93:
- “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
This protects both your readers and your organization.
9. Build a Repeatable FTC/FDA Compliance Workflow
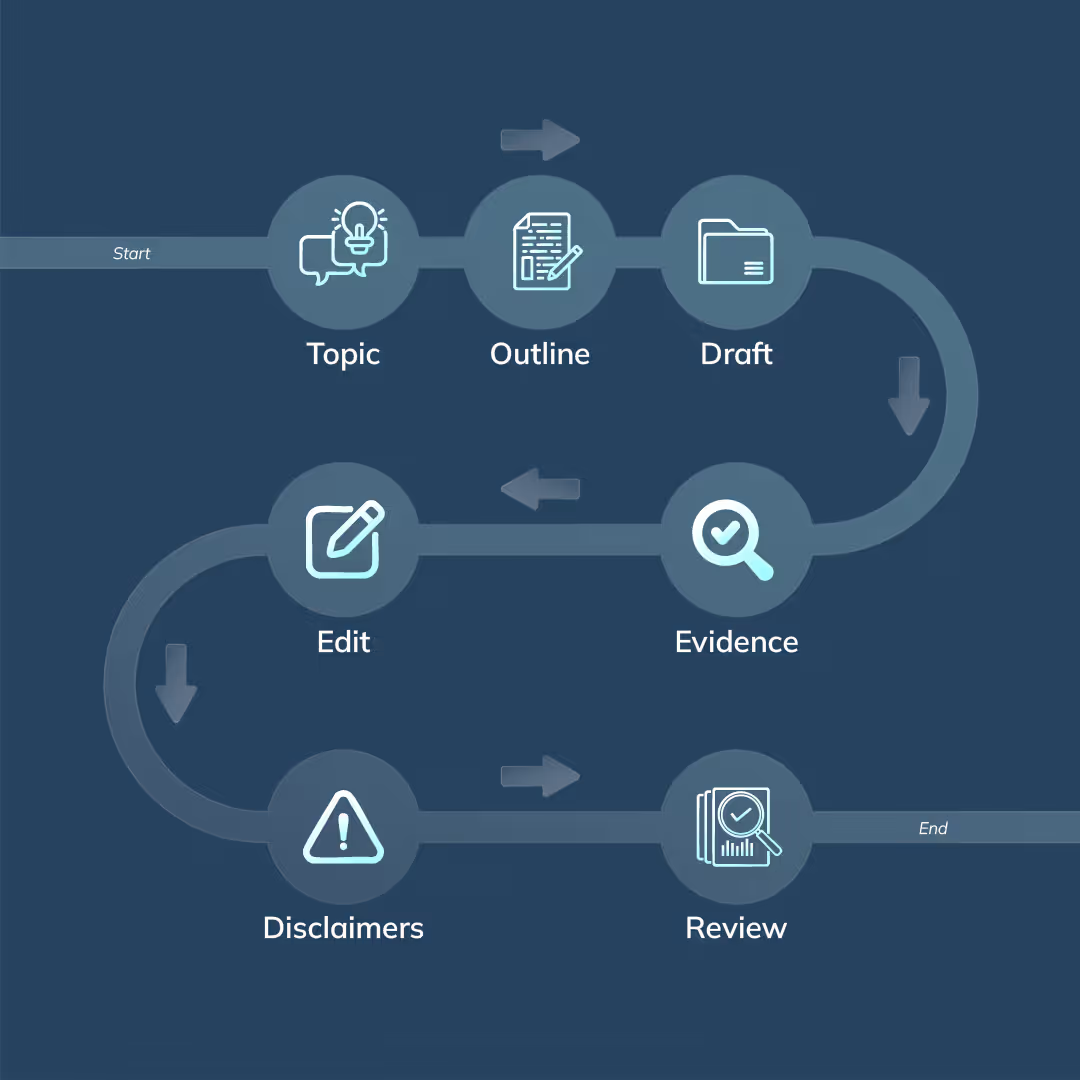
A structured workflow prevents mistakes and keeps your team aligned.
Step 1: Choose Safe Topics
Focus on:
- Physiology
- Biomarkers
- Nutrients
- Lifestyle factors
- Wellness concepts
- General health education
Avoid disease-focused intent.
Step 2: Outline With FDA/FTC Rules in Mind
Plan your structure/function phrasing before drafting.
Step 3: Draft in Clear, Simple Language
Write for comprehension. Avoid jargon.
Step 4: Insert Evidence With Correct Interpretation
Follow FTC evidence standards during citations.
Step 5: Remove or Rephrase Risky Language
Flag disease terms, symptoms, and implied medical advice.
Step 6: Insert Disclaimers
Medical + FDA supplement disclaimers as needed.
Step 7: Final Compliance Review
Especially for:
- Supplement content
- Lab testing content
- Clinical content
- Comparative discussions
One reviewer should be trained in FDA/FTC guidelines.
FDA/FTC Compliant Rewriting Table: Non-Compliant Claims and Their Safe Alternatives
Understanding which health statements violate FDA or FTC guidelines is the first step. This table and interactive tool will help you quickly identify high-risk medical claims and convert them into safe, structure/function language that supports regulatory compliance without losing clarity for readers.
FDA/FTC Health Claim Rewriting Tool for Compliant Healthcare Content
Use the tool below to apply the same compliance rules to your own sentences and generate safe rewrites.
SEO Best Practices for Compliant Healthcare Content
SEO in healthcare requires balancing visibility with regulatory safety. Using compliant language and clear structure helps your content rank while maintaining accuracy, transparency, and consumer trust.
Keyword Strategy
Avoid symptom-targeting keywords.
Choose “support” and “education” style keywords:
- “foods that support gut health”
- “how to support immune resilience”
- “understanding cortisol patterns”
E-E-A-T Optimization
Google prioritizes:
- Experienced authors
- Medical reviewers
- Cited research
- Transparent disclaimers
- High readability
Compliance naturally improves ranking factors.
Content Architecture
Use:
- H2/H3 structure
- Clear subtopics
- Bulleted lists
- Definitions
- Plain-language summaries
This improves both SEO and user trust.
Compliance is essential, but so is visibility. If you want to learn more on how to rank healthcare content while staying compliant, read the full playbook on How to Do SEO for a Healthcare Company.
The Fastest Way to Build Compliant Content Systems
FTC/FDA compliance is not optional in healthcare publishing. It is foundational to safety, trust, and long-term brand protection. The brands that win in 2025 and beyond will be the ones that:
- Understand regulatory frameworks
- Use compliant language
- Present evidence responsibly
- Avoid medical advice
- Keep education separate from promotion
- Build strong review systems
- Hire skilled, experienced content talent
Hiring the right people is the single most powerful lever for improving compliance.
With the top 1% of global healthcare-ready talent from Hire Overseas, you can scale your content operations confidently, reduce regulatory risk, and consistently produce accurate, trustworthy, and compliant health information.
Strengthen your content quality and compliance by upgrading to a team built for accuracy and scale.
FAQs About FTC/FDA Compliant Healthcare Content
What types of healthcare content are most likely to trigger FDA or FTC scrutiny?
Regulators pay the closest attention to content that influences consumer decisions about supplements, diagnostics, devices, or treatments. High-risk formats include product pages, email funnels, advertorials, testimonials, comparison charts, and any content that links symptoms or diseases to specific products—even indirectly.
Do I need clinical studies to support every health-related statement?
Not always. The FTC requires “competent and reliable scientific evidence,” but the level of evidence depends on the strength of the claim. General wellness statements often require less substantiation, while anything approaching measurable benefit, biological effect, or clinical outcome requires stronger human research support.
Are before-and-after photos considered medical claims?
Yes, in many cases. If photos imply measurable improvement, symptom reduction, or a disease-related outcome, the FTC may treat them as advertising claims requiring scientific substantiation. Neutral lifestyle-related imagery is safer but should never imply treatment or prevention.
Can I reference scientific studies without violating compliance rules?
Yes, as long as you do not imply that study findings prove your product or ingredient treats diseases. Use cautious, research-neutral language and avoid extrapolations. Never suggest that a study on one population, dosage, or form guarantees results for consumers.
How do I make sure AI-generated content stays FTC/FDA compliant?
AI tools can unintentionally produce disease claims, prescriptive phrases, or misleading interpretations of research. Always run AI-generated drafts through a human compliance review, check for disease terms, and ensure structure/function phrasing is preserved.
How often should healthcare content be reviewed for compliance?
Most organizations update compliance checklists quarterly and audit high-traffic content at least twice per year. Regulation updates, new FTC enforcement examples, and emerging scientific evidence can all affect content safety over time.
Can outsourced healthcare content teams help with compliance audits and content reviews?
Yes. Many healthcare outsourcing partners provide end-to-end support, including compliance reviews, risk flagging, evidence checks, and rewrites using FDA/FTC-approved language. This ensures content remains safe, accurate, and audit-ready at all times.
Unlock Global Talent with Ease
Hire Overseas streamlines your hiring process from start to finish, connecting you with top global talent.
Unlock Global Talent with Ease
Hire Overseas streamlines your hiring process from start to finish, connecting you with top global talent.